About Celltrion



-
Jan. 2026
Acquisition of the Branchburg Manufacturing Facility (66,000L)

-
Dec. 2023
Celltrion Inc. & Celltrion Healthcare Co., Ltd. merger completed
-
Oct. 2023
Remsima SC (US product name:Zymfentra) received approval by US FDA as novel therapeutics
-
May. 2023
Yuflyma receives approval by US FDA
-
Sep. 2022
Vegzelma receives approval by US FDA
-
Aug. 2022
Vegzelma receives approval by Europe EMA
-
Nov. 2021
Regkirona receives approval by Europe EMA
-
Feb. 2021
Yuflyma receives approval by Europe EMA

-
Aug. 2020
Acquired Primary Care product assets for Asia Pacific markets from Takeda Ltd.
-
Feb. 2020
Reached KRW 1 trillion in annual sales

-
Nov. 2019
Remsima SC receives approval by Europe EMA
-
May. 2019
Expansion of Plant 1 (additional 50,000L) completed
-
Apr. 2019
Linezolid receives approval by US FDA
-
Dec. 2018
Herzuma receives approval by US FDA
-
Nov. 2018
Temixys receives approval by US FDA
Truxima receives approval by US FDA
-
Feb. 2018
Herzuma receives approval by Europe EMA
-
Feb. 2017
Truxima receives approval by Europe EMA

-
Apr. 2016
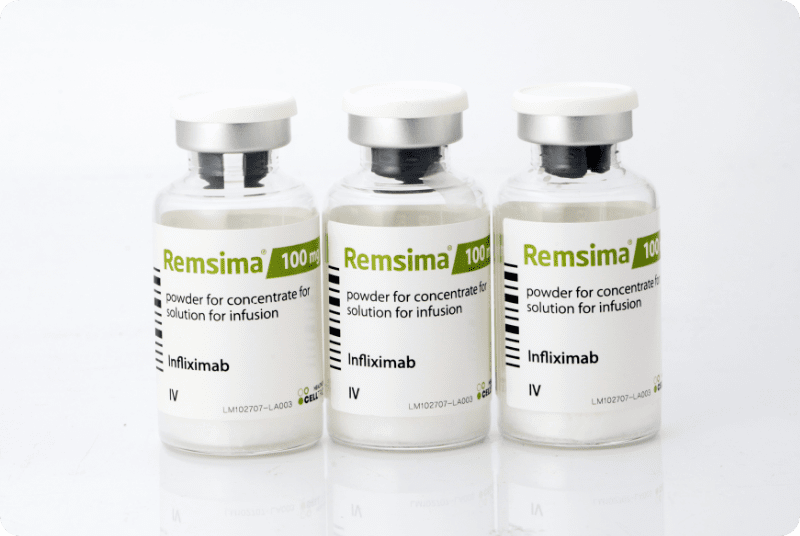
Remsima receives approval by US FDA
-
Jun. 2015
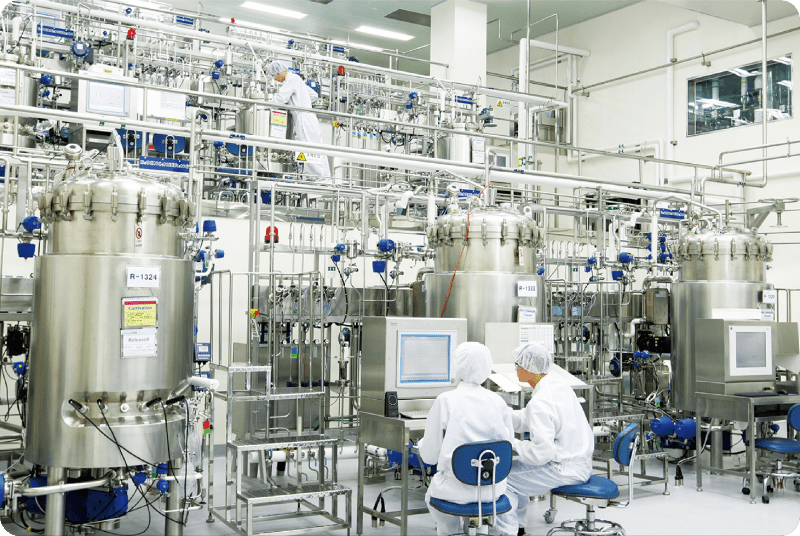
Plant 1 & 2 receive approval by US FDA on all cGMP manufacturing facilities

-
Aug. 2013
Remsima receives approval by Europe EMA
-
Jul. 2012
Remsima receives approval by Korea MFDS

-
Oct. 2011
Plant 2 (90,000L) completed

-
Aug. 2008
Initial Public Offering (IPO)

-
Dec. 2007
Plant 1 receives cGMP facility approval by US FDA

-
Jul. 2005
Plant 1 (50,000L) completed
-
Jun. 2005
Supply agreement with Bristol-Myers Squibb

-
Feb. 2002
Celltrion founded