Formulation: Beyond Conventional Methods, Toward New Therapeutic Strategies
2025.09.29
The term “formulation” in pharmaceuticals refers to the final dosage form designed to deliver active ingredients safely and effectively. Capsules, injectables, ointments, and patches—the many forms of medicine we encounter in daily life—are all types of formulations, with over 190 different types in total.
A drug’s formulation goes beyond its mere “form.” The delivery method affects not only convenience but also therapeutic efficacy, storage stability, and patient response. This is especially critical for biopharmaceuticals, which are structurally complex and highly sensitive to environmental changes, which makes precise formulation design all the more essential.

Based on its extensive experience in formulation development, Celltrion has established a proprietary development framework. Among its key technologies is high-concentration formulation development.
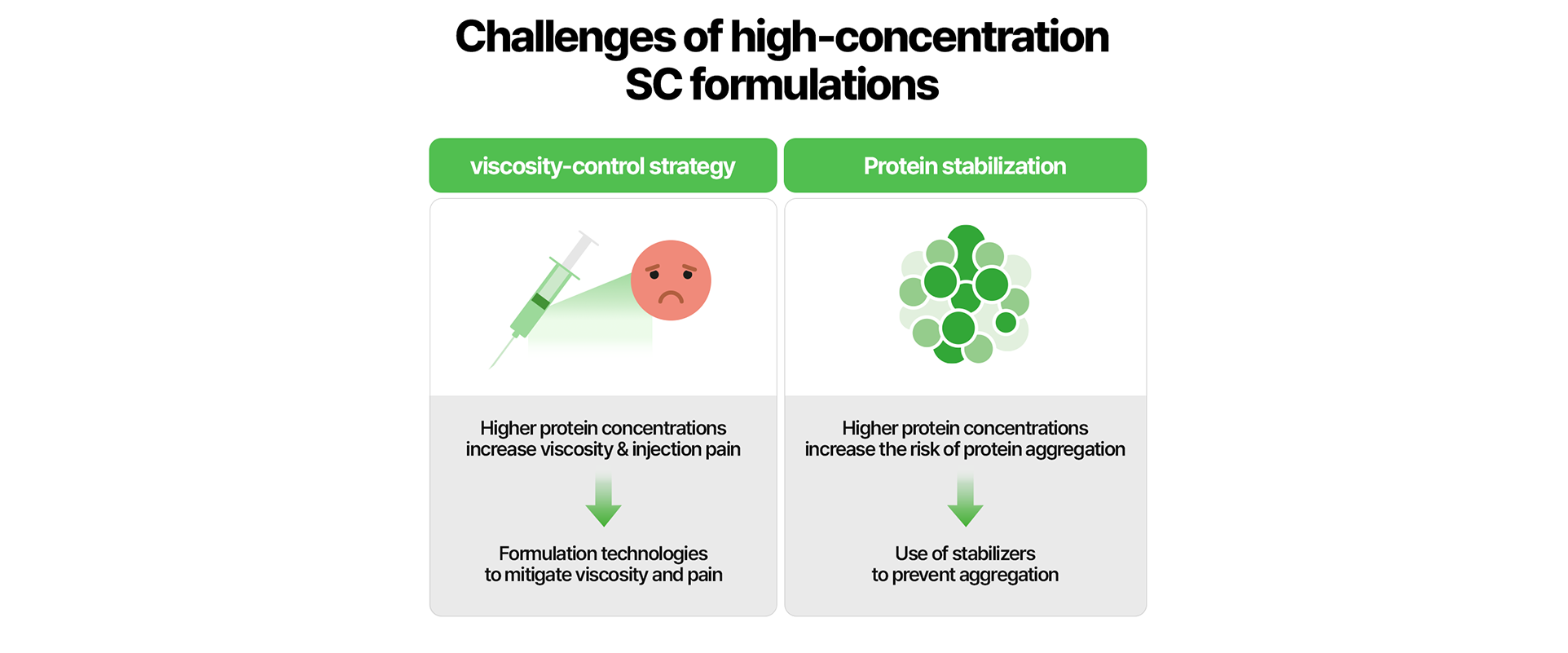
When administered subcutanesouly (SC), high-concentration formulations can lead to injection pain or prolonged injection due to higher antibody concentrations, and can also compromise physical stability through protein aggregation. Overcoming these limitations remains the central challenge in high-concentration formulation development.
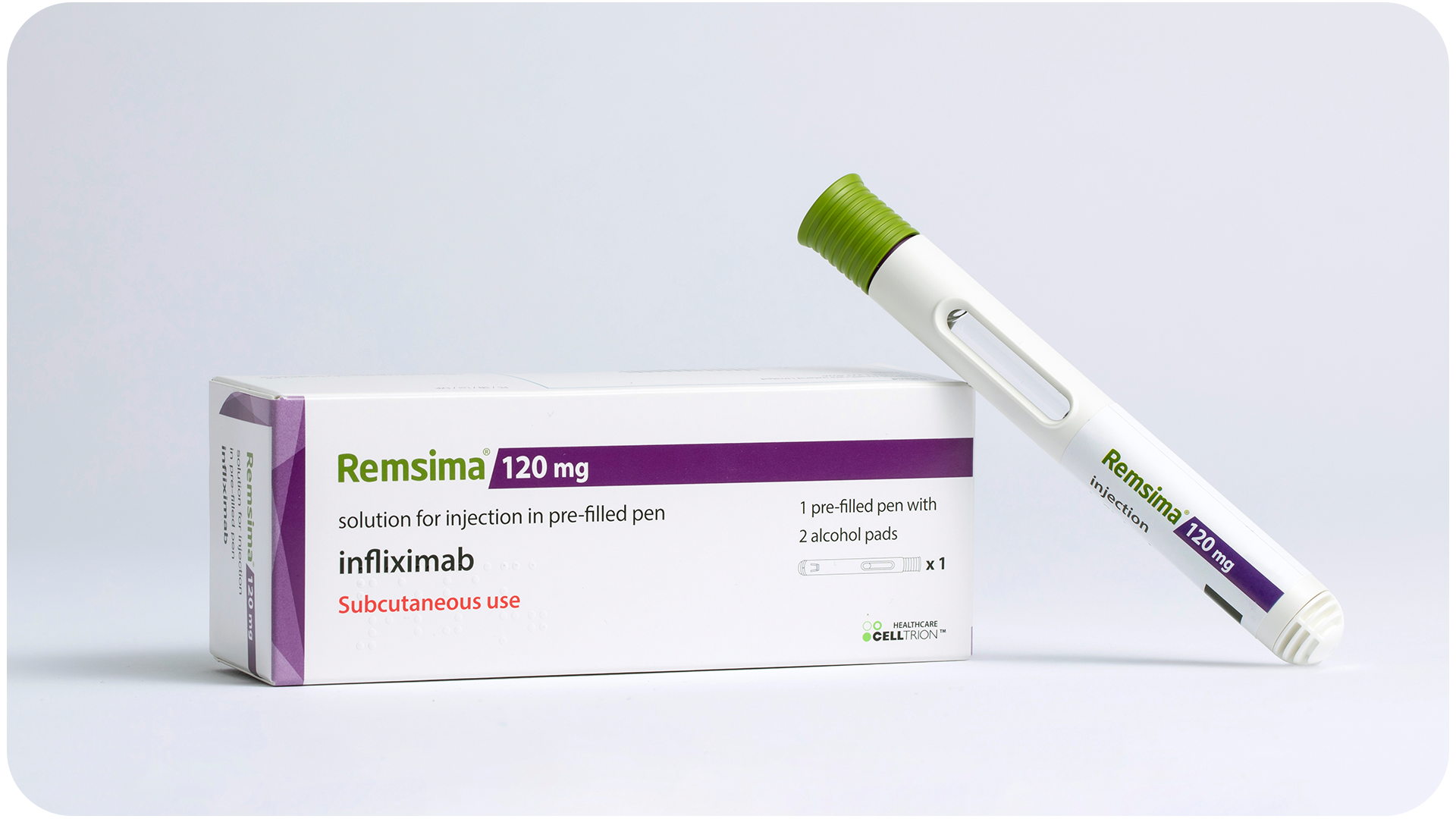
▲ Representative Celltrion high-concentration SC formulation: Remsima SC
Celltrion has also distinguished itself in the conversion of intravenous (IV) formulations into subcutaneous (SC) ones. The most notable example is “Remsima SC,” the SC version of infliximab. Celltrion was the first in the world to successfully make this conversion and obtained approvals in Europe and the U.S., demonstrating its technological leadership. Such formulation innovations foster self-administration, reduce hospital visits, and improve patient convenience while supporting long-term treatment adherence.

Celltrion also develops formulations that take into account physical and chemical compatibility with a range of self-injection devices, including pre-filled syringes and auto-injectors. It has also developed proprietary technology to optimize dosage and injection speed.
Furthermore, Celltrion has implemented a predictive system from the early stage of formulation development to predict drug stability using data-driven analysis. By analyzing factors such as protein structural stability, aggregation potential, and pharmacological properties in advance, it ensures consistent quality even in complex formulations such as high-concentration or freeze-dried types.
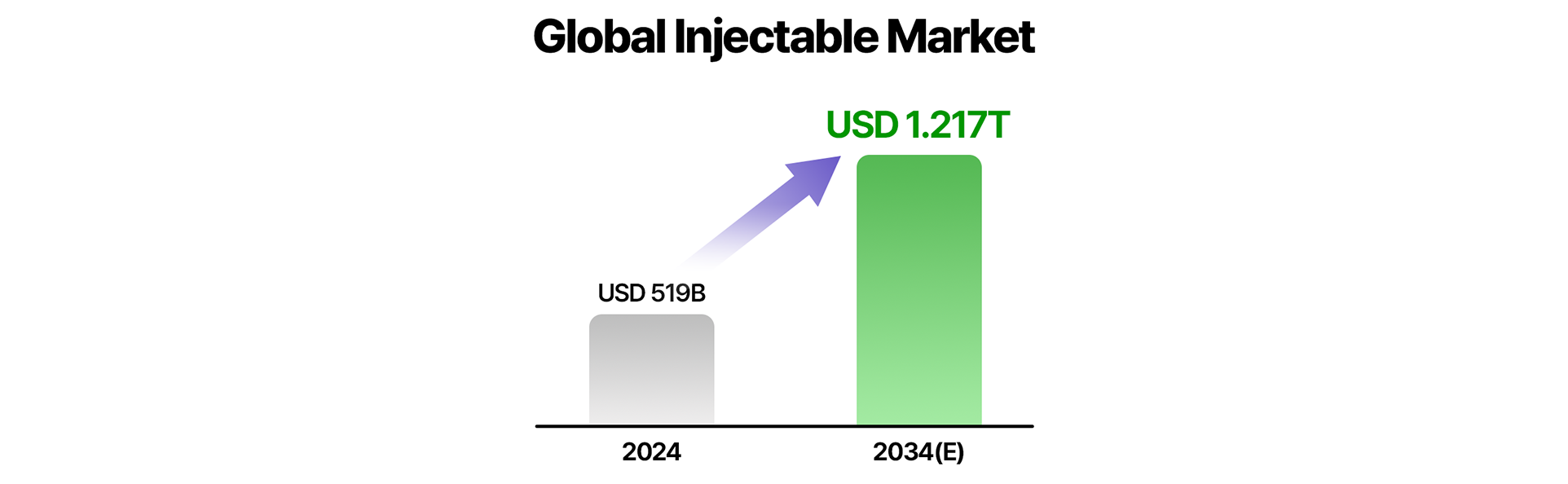
In the global pharma-biotech market, formulation is emerging as a key strategy for differentiation in treatment. The digital health-linked formulation market, including smart syringes, is projected to grow from USD 10.5 billion in 2023 to USD 48.3 billion by 2034. The injectable market, including self-administration devices, is expected to expand from USD 519 billion in 2024 to USD 1.217 trillion by 2034. Alongside high-concentration and extended-release formulations, formulation innovation is becoming a crucial pillar of patient-centered care.

In line with these market trends, Celltrion is proactively advancing formulation technologies, including the incorporation of human hyaluronidase, the optimization of high-concentration formulations, and the design of customized self-injection devices. It also employs a quantitative, data-driven formulation design system that takes into account patient environments and real-world usage conditions.

Going forward, Celltrion will continue to view formulation as its core competitive strength, spearheading the development of strategic medicines that deliver both convenience and therapeutic efficacy.
* Credence Research, Apr 12, 2025
** GlobeNewswise, Apr 10, 2025